Accelerated evaporation of water on graphene oxide†
Abstract
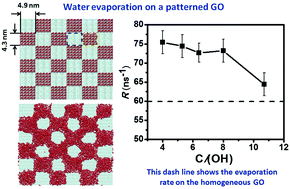
Using molecular dynamics simulations, we show that the evaporation of nanoscale volumes of water on patterned graphene oxide is faster than that on homogeneous graphene oxide. The evaporation rate of water is insensitive to variation in the oxidation degree of the oxidized regions, so long as the water film is only distributed on the oxidized regions. The evaporation rate drops when the water film spreads onto the unoxidized regions. Further analysis showed that varying the oxidation degree observably changed the interaction between the outmost water molecules and the solid surface, but the total interaction for the outmost water molecules only changed a very limited amount due to the correspondingly regulated water–water interaction when the water film is only distributed on the oxidized regions. When the oxidation degree is too low and some unoxidized regions are also covered by the water film, the thickness of the water film decreases, which extends the lifetime of the hydrogen bonds for the outmost water molecules and lowers the evaporation rate of the water. The insensitivity of water evaporation to the oxidation degree indicates that we only need to control the scale of the unoxidized and oxidized regions for graphene oxide to regulate the evaporation of nanoscale volumes of water.


 Please wait while we load your content...
Please wait while we load your content...