Thermodynamic and morphological characterization of Turing patterns in non-isothermal reaction–diffusion systems
Abstract
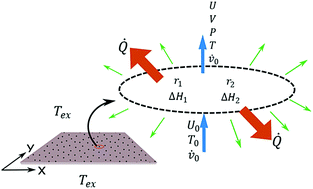
The effect of temperature on the bifurcation diagram and Turing instability domain under non isothermal conditions is studied in the reversible Gray–Scott model. After adding the energy balance to the cubic autocatalytic model, the thermostat temperature and heat transfer coefficient are used as control parameters in the Turing pattern formation. The patterns obtained in the domain of the thermal parameter are characterized by quantifying the overall entropy generation rate and two topological indices; Shannon entropy and Minkowski functionals. The results show that it is possible to induce transitions between Turing patterns of different morphologies by regulating the temperature, and that these transitions take place at a lower entropy generation value compared to other parameters, such as kinetic constants and reactant fluxes. Finally, a correlation between entropy generation and topological indices shows that a difference between direct and inverse patterns is mainly morphological and not energetic.


 Please wait while we load your content...
Please wait while we load your content...