First-principles computation of electron transfer and reaction rate at a perovskite cathode for hydrogen production†
Abstract
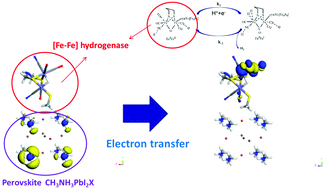
The focus of this research is on the electron transfer and its reaction rate at the perovskite cathode of a photoelectrochemical cell for hydrogen production. By employing the density functional theory (DFT), the electron density, projected density of states (PDOS), electron distribution and electron transfer path between [Fe–Fe] hydrogenase and the perovskite cathode can be obtained. Simulation results show that the perovskite cathode is better than traditional cathodes for hydrogen production. Before transmission to the [Fe–Fe] hydrogenase, electron clouds mainly aggregate at the periphery of amine molecules. Simulations also show that the key to hydrogen production at the perovskite structure lies in the organic molecules. Electrons are transferred to the hydrocarbon structural chain before reaching the Fe atoms. The Rice, Ramsperger, Kassel and Marcus (RRKM) theory was used to predict the reaction rates at different temperatures. It was found that the reaction rates are in good agreement with the experimental results. This research provides more physical insight into the electron transfer mechanism during the hydrogen production process.


 Please wait while we load your content...
Please wait while we load your content...