Electrophilicity of oxalic acid monomer is enhanced in the dimer by intermolecular proton transfer†
Abstract
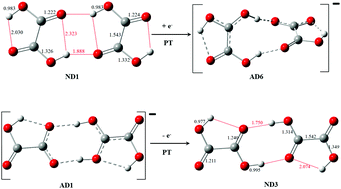
We have analyzed the effect of excess electron attachment on the network of hydrogen bonds in the oxalic acid dimer (OA)2. The most stable anionic structures may be viewed as complexes of a neutral hydrogenated moiety HOA˙ coordinated to an anionic deprotonated moiety (OA–H)−. HOA˙ acts as a double proton donor and (OA–H)− as a double proton acceptor. Thus the excess electron attachment drives intermolecular proton transfer. We have identified several cyclic hydrogen bonded structures of (OA)2−. Their stability has been analyzed in terms of the stability of the involved conformers, the energetic penalty for deformation of these conformers to the geometry of the dimer, and the two-body interaction energy between the deformed HOA˙ and (OA–H)−. There are at least seven isomers of (OA)2− with stabilization energies in the range of 1.26–1.39 eV. These energies are dominated by attractive two-body interaction energies. The anions are vertically bound electronically by 3.0–3.4 eV and adiabatically bound by at least 1.6 eV. The computational predictions are consistent with the anion photoelectron spectrum of (OA)2−. The spectrum consists of a broad feature, with an onset of 2.5 eV and spanning to 4.3 eV. The electron vertical detachment energy (VDE) is assigned to be 3.3 eV.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...