Gas-phase vs. material-kinetic limits on the redox response of nonstoichiometric oxides†
Abstract
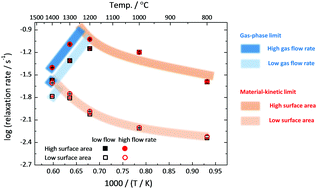
Cerium dioxide, CeO2−δ, remains one of the most attractive materials under consideration for solar-driven thermochemical production of chemical fuels. Understanding the rate-limiting factors in fuel production is essential for maximizing the efficacy of the thermochemical process. The rate of response is measured here via electrical conductance relaxation methods using porous ceria structures with architectural features typical of those employed in solar reactors. A transition from behavior controlled by material surface reaction kinetics to that controlled by sweep-gas supply rates is observed on increasing temperature, increasing volume specific surface area, and decreasing normalized gas flow rate. The transition behavior is relevant not only for optimal reactor operation and architectural design of the material, but also for accurate measurement of material properties.


 Please wait while we load your content...
Please wait while we load your content...