The role of conformational heterogeneity in regulating the apoptotic activity of BAX protein†
Abstract
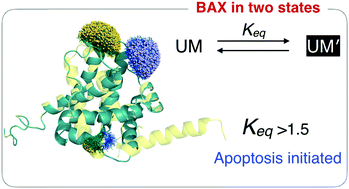
While activation of BAX is required for initiating mitochondria-mediated apoptosis, the underlying mechanisms remain unsettled. We studied conformations of BAX protein using pressure- and temperature-resolved ESR techniques and obtained the thermodynamic properties of the conformations. We show that inactive BAX is structurally heterogeneous and exists in equilibrium between two major populations of the conformations, UM and UM′, of which the former is thermodynamically favored at room temperature. An increase in the population of UM′, induced by either pressure or point mutations of BAX, renders BAX susceptible to oligomerization, which leads to cell death. This study uncovers the biological significance of BAX conformations and shows that the pro-apoptotic activity of BAX can be triggered by altering the equilibrium between the two states. It suggests that therapeutic intervention may focus on shifting the balance in the conformational heterogeneity.


 Please wait while we load your content...
Please wait while we load your content...