Oxygen thermomigration in acceptor-doped perovskite
Abstract
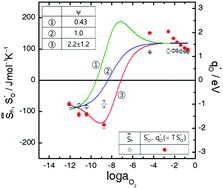
The recent proposal of possible oxygen-thermomigration as a plausible mechanism for unipolar resistive switching of oxide memristors is now widely employed in modelling or simulating their memristive function on the grounds of the conventional picture that the mobile component O is always thermophobic, with its reduced heat-of-transport being equal to its migrational enthalpy (qO* = ΔHm > 0). At 1000 °C, we measured the thermomigration of mobile-component O in a prototype memristive perovskite, CaTi0.90Sc0.10O2.95+δ, across its near-stoichiometric regime (δ ≈ 0), where oxygen vacancy concentration is essentially fixed by doping acceptor impurities (i.e., 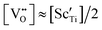 ). It has been found that the reduced heat-of-transport of mobile O (qO*) varies systematically in the range of −2 < qO*/eV ≤ +2, as the composition varies from hypo-(δ < 0) to hyper-stoichiometry (δ > 0), exhibiting a sign reversal or thermomigration direction change from thermophilic (qO* < 0) to thermophobic (qO* > 0). This sign-reversal is attributed to the change in the electronic ambipolar company of
). It has been found that the reduced heat-of-transport of mobile O (qO*) varies systematically in the range of −2 < qO*/eV ≤ +2, as the composition varies from hypo-(δ < 0) to hyper-stoichiometry (δ > 0), exhibiting a sign reversal or thermomigration direction change from thermophilic (qO* < 0) to thermophobic (qO* > 0). This sign-reversal is attributed to the change in the electronic ambipolar company of 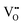 from electrons to holes crossing the stoichiometric composition δ = 0. The numerical data for qO* together with the measurement details are reported.
from electrons to holes crossing the stoichiometric composition δ = 0. The numerical data for qO* together with the measurement details are reported.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...