Monocyclic aromatic compounds BnRgn(n−2)+ of boron and rare gases†
Abstract
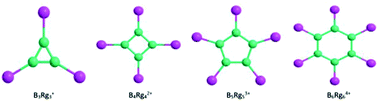
The monocyclic compounds (BRg)3+(D3h), (BRg)42+(D4h), (BRg)53+(D5h) and (BRg)64+(D6h) formed between boron and rare gases Rg (He–Rn) are theoretically predicted to be stable structures and have π-aromaticity with a delocalized nc-2e π-system. For heavier rare gases Ar–Rn, the B–Rg bond energy is quite high and ranges from 15 to 96 kcal mol−1, increasing with the ring size and the atomic number of rare gases; the B–Rg bond length is close to the sum of covalent radii of B and Rg atoms; NBO and AIM analyses show that the B–Rg bonds for Ar–Rn have a typical covalent character. The B–Rg bond is stabilized mainly by σ-donation from the valence p orbital of Rg to the vacant valence orbital of the boron ring. We searched for a large number of isomers for the systems of Ar and found that the titled monocyclic compounds (BAr)3+(D3h), (BAr)42+(D4h) and (BAr)53+(D5h) should be global energy minima. For (BAr)64+ the global energy minimum is an octahedral caged structure, but the titled monocyclic compound is the secondary stable local energy minimum. The energy and thermodynamic stability of the ring BnRgn(n−2)+ cations indicate that these rare gas compounds may be viable species in experiments.


 Please wait while we load your content...
Please wait while we load your content...