Theoretically derived mechanisms of HPALD photolysis in isoprene oxidation†
Abstract
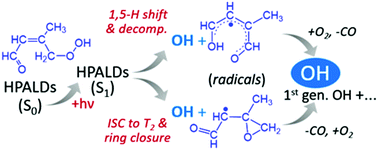
In this work we identified and theoretically quantified two photolysis mechanisms of HPALDs (hydroperoxy aldehydes) that result from the isomerization of peroxy radicals in the atmospheric oxidation of isoprene at low/moderate NOx. As a first photolysis mechanism, we show that a fraction of the initially excited S1-state HPALDs isomerizes by a near-barrierless 1,5 H-shift at a rate approaching 1012 s−1 – competing with the ∼equally fast intersystem crossing to the T2 triplet state – forming an unstable biradical that spontaneously expels an OH (hydroxyl) radical. A second mechanism is shown to proceed through the activated T2 triplet biradical – formed from S1 – undergoing a concerted ring-closure and OH-expulsion, yielding an oxiranyl-type co-product radical that quickly ring-opens to enoxy radicals. In both mechanisms, subsequent chemistry of the co-product radicals yields additional first-generation OH. The combined HPALD-photolysis quantum yield by these two mechanisms – which may not be the only photolysis routes – is estimated at 0.55 and the quantum yield of OH generation at 0.9, in fair accordance with experimental data on an HPALD proxy (Wolfe et al., Phys. Chem. Chem. Phys., 2012, 14, 7276–7286).

 Please wait while we load your content...
Please wait while we load your content...