Preexisting domain motions underlie protonation-dependent structural transitions of the P-type Ca2+-ATPase
Abstract
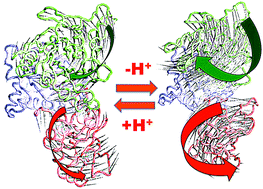
We have performed microsecond molecular dynamics (MD) simulations to determine the mechanism for protonation-dependent structural transitions of the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA), one of the most prominent members of the large P-type ATPase superfamily that transports ions across biological membranes. The release of two H+ from the transport sites activates SERCA by inducing a structural transition between low (E2) and high (E1) Ca2+-affinity states (E2-to-E1 transition), but the structural mechanism by which transport site deprotonation facilitates this transition is unknown. We performed microsecond all-atom MD simulations to determine the effects of transport site protonation on the structural dynamics of the E2 state in solution. We found that the protonated E2 state has structural characteristics that are similar to those observed in crystal structures of E2. Upon deprotonation, a single Na+ ion rapidly (<10 ns) binds to the transmembrane transport sites and induces a kink in M5, disrupts the M3–M5 interface, and increases the mobility of the M3/A-M3 linker. Principal component analysis showed that counter-rotation of the cytosolic N–A domains about the membrane normal axis, which is the primary motion driving the E2-to-E1 transition, is present in both protonated and deprotonated E2 states; however, protonation-dependent structural changes in the transmembrane domain control the hierarchical organization and amplitude of this motion. We propose that preexisting rigid-body domain motions underlie structural transitions of SERCA, where the functionally important directionality is preserved while transport site protonation controls the dominance and amplitude of motion to shift the equilibrium between the E1 and E2 states. We conclude that ligand-induced modulation of preexisting domain motions is likely a common theme in structural transitions of the P-type ATPase superfamily.


 Please wait while we load your content...
Please wait while we load your content...