Insights into acid dissociation of HCl and HBr with internal electric fields†
Abstract
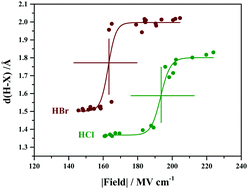
The electric field experienced by an acid molecule in the acid–water cluster depends on its local environment comprising of surrounding water molecules. A critical field of about 193 and 163 MV cm−1 is required for the dissociation of HCl and HBr, respectively, and is associated with the arrangement of water molecules around the acid. The critical field required for dissociation of isolated HCl and HBr is 510 and 462 MV cm−1, respectively. Hence the solvation of the proton and the halide anion by water molecules substantially lowers the critical electric field by about 300 MV cm−1, relative to vacuum.
- This article is part of the themed collection: New Frontiers in Indian Research


 Please wait while we load your content...
Please wait while we load your content...