Simultaneous covalent and noncovalent carbon nanotube/Ag3PO4 hybrids: new insights into the origin of enhanced visible light photocatalytic performance
Abstract
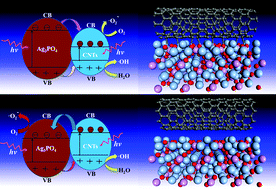
Understanding the interfacial interaction is of paramount importance for rationally designing carbon nanomaterial-based hybrids with optimal performance for electronics, optoelectronics, sensing, advanced energy conversion and storage. Here, we firstly reveal that both covalent and noncovalent interactions simultaneously exist in carbon nanotube (CNT)/Ag3PO4 hybrids by studying systematically the electronic and optical properties to elucidate the mechanism of their enhanced photocatalytic performance. Metallic CNT(9,0) may chemically or physically interact with the Ag3PO4(100) surface depending on its relative orientations, whereas semiconducting CNT(10,0) can only noncovalently functionalize Ag3PO4. The C–Ag bond in the covalently bonded hybrid and type-II, staggered, band alignment in noncovalent hybrids lead to a robust separation of photoexcited charge carriers between two constituents, thus enhancing the photocatalytic activity. The small band gap makes the CNT/Ag3PO4 hybrids absorb sunlight from the ultraviolet to infrared region. Moreover, CNTs are not only effective sensitizers, but also highly active co-catalysts in hybrids. The results can be rationalized by the available experiments, thereby partly resolving a debate on the interpretation of the experimental results, and paving the way for developing highly efficient carbon-based nanophotocatalysts.


 Please wait while we load your content...
Please wait while we load your content...