Prediction of strong O–H/M hydrogen bonding between water and square-planar Ir and Rh complexes†
Abstract
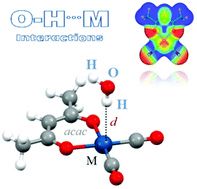
Intermolecular O–H/M interactions, between a water molecule and square-planar acac complexes ([M(acac)L2]), with different types of L ligands (en, H2O, CO, CN−, and OH−) and different types of metal atoms (Ir(I), Rh(I), Pt(II), and Pd(II)) were studied by high level ab initio calculations. Among the studied neutral complexes, the [Pd(acac)(CN)(CO)] complex forms the weakest interaction, −0.62 kcal mol−1, while the [Ir(acac)(en)] complex forms the strongest interaction, −9.83 kcal mol−1, which is remarkably stronger than the conventional hydrogen bond between two water molecules (−4.84 kcal mol−1).
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...