A first-principles study on the adsorption of ethylenediamine on Ge(100)
Abstract
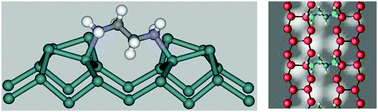
We have performed density functional theory (DFT) calculations of the atomic and electronic structures of ethylenediamine on Ge(100). The two amine groups in ethylenediamine can interact with germanium surface atoms through a N–H dissociative nucleophilic reaction and/or N-dative bonding with an electron-deficient down Ge atom. Of the monodentate and row-bridged bidentate structures that formed, the dative-bonded configurations were found to be more stable than the NH dissociative adsorption structures. The formation of row-bridged bidentate, structures is more favorable than that of on-top or end-bridged structures. In simulated STM images, the three types of row-bridged adsorption structure have characteristic features, and the row-bridged dative-bonded configuration gives rise to features due to both adsorbed ethylenediamine molecules and the underlying Ge atoms.


 Please wait while we load your content...
Please wait while we load your content...