Controlling the termination and photochemical reactivity of the SrTiO3(110) surface†
Abstract
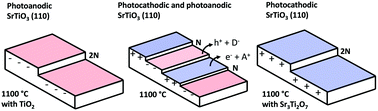
SrTiO3(110) orientated crystals have been heated to temperatures between 1000 °C and 1200 °C in air, alone or in the presence of powder reservoirs of TiO2 or Sr3Ti2O7. In these conditions, the surface is terminated by two types of atomically flat terraces. One has a relatively higher surface potential and promotes the photochemical reduction of silver (it is photocathodic) and the other has a relatively lower surface potential and promotes the photochemical oxidation of lead (it is photoanodic). Measurements of the step heights between the terraces indicate that the surfaces with different properties have different terminations. By adjusting the time and temperature of the anneal, and in some cases including reservoirs of TiO2 or Sr3Ti2O7, it is possible to change the surface area fraction from 98% photocathodic to 100% photoanodic. The surface is more photocathodic when the annealing temperature is lower, the duration shorter, and if Sr3Ti2O7 is present. The surface is more photoanodic if the temperature is higher, the annealing duration longer, and if TiO2 is present. The results make it possible to control the surface potential and the ratio of photocathodic to photoanodic area on the SrTiO3(110) surface.


 Please wait while we load your content...
Please wait while we load your content...