How is charge transport different in ionic liquids? The effect of high pressure†
Abstract
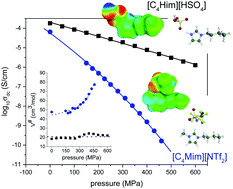
Modern ionic liquids (ILs) are considered green solvents for the future applications due to their inherited advantages and remarkable transport properties. One of the ubiquitous properties of ILs is their intrinsic ionic conductivity. However, understanding of the super-Arrhenius behavior of the ionic conductivity process at elevated pressure still remains elusive and crucial in glass science. In this work, we investigate the ion transport properties of 1-butyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imide: [C4mim][NTf2], 1-butylimidazolium bis[(trifluoromethyl)-sulfonyl]imide: [C4Him][NTf2] and 1-butylimidazolium hydrogen sulfate: [C4Him][HSO4] ILs in the supercooled liquid state using dielectric spectroscopy at ambient and high pressure. We present the experimental data in the dynamic window of the conductivity formalism to examine the charge transport properties. The frequency-dependent ionic conductivity data have been analyzed using the time–temperature superposition principle. In the Arrhenius diagram, the thermal evolution of the dc-conductivity reveals similar temperature dependence for both protic and aprotic ILs thus making it difficult to distinguish the ion dynamics. However, our results demonstrate the key role of high pressure that unambiguously separates the charge transport properties of protic ILs from aprotic ones through the apparent activation volume parameter. We also highlight that the activation volume can be employed to assess the information connecting the ability of ionic systems to form H-bond networks and the impact of proton transfer involved in the conduction process.


 Please wait while we load your content...
Please wait while we load your content...