Role of polar side chains in Li+ coordination and transport properties of polyoxetane-based polymer electrolytes†
Abstract
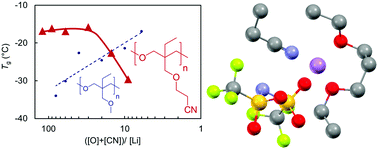
Lithium ion conducting polymer electrolytes (PEs) have been the subject of intense research for lithium metal battery applications. Here, we investigate the effects of polar side chains on Li+ coordination and ionic transport properties to gain insights for improving the insufficient conductivity of traditional ether-based solid PEs. Poly(trimethyleneoxide)-based (or polyoxetane-based) polymers with ether or nitrile groups were synthesized by ring-opening polymerization. The thermal, ionic transport, and electrochemical properties and the local structure of Li+ coordination were studied in the presence of lithium bis(trifluoromethanesulfonyl)amide (LiTFSA). The glass transition temperature (Tg) of the PEs with ether side chains increased with increasing LiTFSA content, whereas the PEs with the nitrile functionality showed the opposite trend at higher salt concentrations. In addition to the unique trend for the Tg values of the PEs in the presence of LiTFSA, the nitrile groups played pivotal roles as coordination sites for Li+ ions in the first coordination shell and as a polar medium to increase the permittivity of the PEs. These characteristics of the nitrile groups can endow PEs with improved ionic transport properties.


 Please wait while we load your content...
Please wait while we load your content...