Formation of atmospheric molecular clusters consisting of sulfuric acid and C8H12O6 tricarboxylic acid†
Abstract
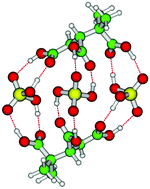
Using computational methods, we investigate the formation of atmospheric clusters consisting of sulfuric acid (SA) and 3-methyl-1,2,3-butanetricarboxylic acid (MBTCA), identified from α-pinene oxidation. The molecular structure of the clusters is obtained using three different DFT functionals (PW91, M06-2X and ωB97X-D) with the 6-31++G(d,p) basis set and the binding energies are calculated using a high level DLPNO-CCSD(T)/Def2-QZVPP method. The stability of the clusters is evaluated based on the calculated formation free energies. The interaction between MBTCA and sulfuric acid is found to be thermodynamically favourable and clusters consisting of 2–3 MBTCA and 2–3 SA molecules are found to be particularly stable. There is a large stabilization of the cluster when the amount of sulfuric acid–carboxylic acid hydrogen bonded interactions is maximized. The reaction free energies for forming the (MBTCA)2–3(SA)2–3 clusters are found to be similar in magnitude to those of the formation of the sulfuric acid–dimethylamine cluster. Using cluster kinetics calculations we identify that the growth of the clusters is essentially limited by a weak formation of the largest clusters studied, implying that other stabilizing vapours are required for stable cluster formation and growth.


 Please wait while we load your content...
Please wait while we load your content...