The mechanism of oxidation in chromophore maturation of wild-type green fluorescent protein: a theoretical study†
Abstract
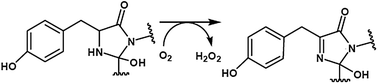
Oxidation is viewed as the second and rate-limiting step in the chromophore maturation process of the wild-type green fluorescent protein (GFP) under aerobic conditions. Molecular oxygen is the necessary oxidant for GFP chromophore biosynthesis. In this study, density functional theory (DFT) calculations were employed to study the mechanism of oxidation. Our results indicate that the deprotonation of the Tyr66 α-carbon is probably the rate-limiting step in the oxidation step. Electron transfer from the enolate form of the five-membered heterocycle (EFMH) to molecular oxygen, generating the triplet radical complex [EFMH˙⋯O2−˙]T, is an important step. This complex undergoes intersystem crossing to form an open-shell singlet diradical complex before it forms the closed-shell singlet hydroperoxy adduct. The formation of the hydroperoxy adduct is a proton-coupled electron transfer process. The energy barrier of H2O2 elimination is 16.5 kcal mol−1. The oxidation product IFMH⋯H2O2 that we discovered is a hydroxylated cyclic imine structure, which is consistent with the crystal structure trapped in the colorless Y66L variant. The relative energy of the oxidation product is −48.7 kcal mol−1, which is in accordance with the experimental observation that the thermodynamically unfavourable cyclized product is trapped by oxidation. The results herein support the cyclization–oxidation–dehydration mechanism for the chromophore maturation of GFP.


 Please wait while we load your content...
Please wait while we load your content...