Maltose-binding protein effectively stabilizes the partially closed conformation of the ATP-binding cassette transporter MalFGK2†
Abstract
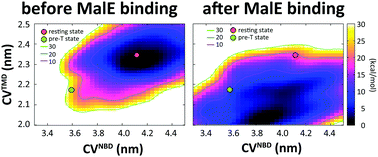
Maltose transporter MalFGK2 is a type-I importer in the ATP-binding cassette (ABC) transporter superfamily. Upon the binding of its periplasmic binding protein, MalE, the ATPase activity of MalFGK2 can be greatly enhanced. Crystal structures of the MalFGK2–MalE–maltose complex in a so-called “pretranslocation” (“pre-T”) state with a partially closed conformation suggest that the formation of this MalE-stabilized intermediate state is a key step leading to the outward-facing catalytic state. On the contrary, crosslinking and fluorescence studies suggest that ATP binding alone is sufficient to promote the outward-facing catalytic state, thereby doubting the role of MalE binding. To clarify the role of MalE binding and to gain deeper understanding of the molecular mechanisms of MalFGK2, we calculated the free energy surfaces (FESs) related to the lateral motion in the presence and absence of MalE using atomistic metadynamics simulations. The results showed that, in the absence of MalE, laterally closing motion was energetically forbidden but, upon MalE binding, more closed conformations similar to the pre-T state become more stable. The significant effect of MalE binding on the free energy landscapes was in agreement with crystallographic studies and confirmed the important role of MalE in stabilizing the pre-T state. Our simulations also revealed that the allosteric effect of MalE stimulation originates from the MalE-binding-promoted vertical motion between MalF and MalG cores, which was further supported by MD simulation of the MalE-independent mutant MalF500.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...