Evidence for photosensitised hydrogen production from water in the absence of precious metals, redox-mediators and co-catalysts†
Abstract
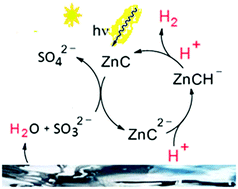
The water-soluble zinc porphyrin complex Zn(TPPS)4− with TPPS = tetrakis-(4-sulfonatophenyl)porphyrin surprisingly was found to produce significant amounts of hydrogen from aqueous sulfite or amine solutions under visible-light exposure without requiring any other components such as electron relays or additional proton reduction catalysts. Although the production rates and total amounts of chemically stored fuel obtained under these conditions are still much too low to be relevant for practical applications, the background of this unprecedented observation was further studied in its own right. Since the central metal zinc is unlikely to be involved in proton-coupled electron transfer steps upon long-wavelength irradiation and the process does not seem to be much affected by variations of the electron donor added, the mechanism of photocatalytic H2 release is suggested to involve previously neglected redox features of the in situ generated hydroporphyrin ligand system in aqueous solution.


 Please wait while we load your content...
Please wait while we load your content...