Toward accurately modeling N-methylated cyclic peptides†
Abstract
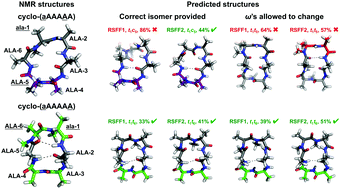
Cyclic peptides have unique properties and can target protein surfaces specifically and potently. N-Methylation provides a promising way to further optimize the pharmacokinetic and structural profiles of cyclic peptides. The capability to accurately model structures adopted by N-methylated cyclic peptides would facilitate rational design of this interesting and useful class of molecules. We apply molecular dynamics simulations with advanced enhanced sampling methods to efficiently characterize the structural ensembles of N-methylated cyclic peptides, while simultaneously evaluating the overall performance of several simulation force fields. We find that one of the residue-specific force fields, RSFF2, is able to recapitulate experimental structures of the N-methylated cyclic peptide benchmarks tested here when the correct amide isomers are used as initial configurations and enforced during the simulations. Thus, using our simulation approach, it is possible to accurately and efficiently predict the structures of N-methylated cyclic peptides if sufficient information is available to determine the correct amide cis/trans configuration. Moreover, our results suggest that, upon further optimization of RSFF2 to more reliably predict cis/trans isomers, molecular dynamics simulations will be able to de novo predict N-methylated cyclic peptides in the near future, strongly motivating such continued optimization.


 Please wait while we load your content...
Please wait while we load your content...