Quantitative assessment of kosmotropicity of hydrated ionic liquids by nuclear magnetic resonance†
Abstract
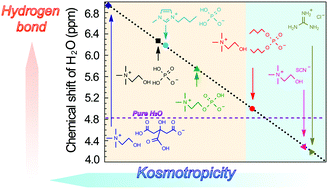
Nuclear magnetic resonance studies revealed that the chemical shift of H2O in hydrated ionic liquids varied with their component ions. The variation reflected the formation of hydrogen bonding networks between ions and water molecules. The chemical shift relative to bulk water was used to quantitatively assess the kosmotropicity.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...