Thermodynamic and molecular origin of interfacial rate enhancements and endo-selectivities of a Diels–Alder reaction†
Abstract
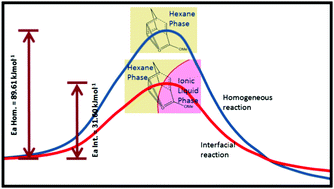
Organic reactions in general display large rate accelerations when performed under interfacial conditions, such as on water or at ionic liquid interfaces. However, a clear picture of the physicochemical factors responsible for this large rate enhancements is not available. To gain an understanding of the thermodynamic and molecular origin of these large rate enhancements, we performed a Diels–Alder reaction between cyclopentadiene and methyl acrylate at ionic liquid/n-hexane interfaces. This study describes, for the first time, a methodology for the calculation of the activation parameters of an interfacial reaction. It has been seen that the energy of activation for an interfacial reaction is much smaller than that of the corresponding homogeneous reaction, resulting into the large rate acceleration for the interfacial reaction. Furthermore, the study describes the effects of the alkyl chain length of ionic liquid cations, the extent of heterogeneity, and the polarity of ionic liquids on the rate constants and stereoselectivity of the reaction.


 Please wait while we load your content...
Please wait while we load your content...