Molecular dynamics simulation of the structure and interfacial free energy barriers of mixtures of ionic liquids and divalent salts near a graphene wall
Abstract
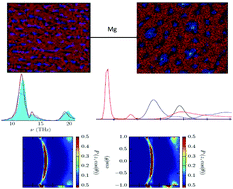
A molecular dynamics study of mixtures of 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIm][BF4]) with magnesium tetrafluoroborate (Mg[BF4]2) confined between two parallel graphene walls is reported. The structure of the system is analyzed by means of ionic density profiles, lateral structure of the first layer close to the graphene surface and angular orientations of imidazolium cations. Free energy profiles for divalent magnesium cations are calculated using two different methods in order to evaluate the height of the potential barriers near the walls, and the results are compared with those of mixtures of the same ionic liquid and a lithium salt (Li[BF4]). Preferential adsorption of magnesium cations is analyzed using a simple model and compared to that of lithium cations, and vibrational densities of states are calculated for the cations close to the walls analyzing the influence of the graphene surface charge. Our results indicate that magnesium cations next to the graphene wall have a roughly similar environment to that in the bulk. Moreover, they face higher potential barriers and are less adsorbed on the charged graphene walls than lithium cations. In other words, magnesium cations have a more stable solvation shell than lithium ones.


 Please wait while we load your content...
Please wait while we load your content...