Determining crystal phase purity in c-BP through X-ray absorption spectroscopy†
Abstract
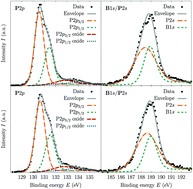
We employ X-ray absorption near-edge spectroscopy at the boron K-edge and the phosphorus L2,3-edge to study the structural properties of cubic boron phosphide (c-BP) samples. The X-ray absorption spectra are modeled from first-principles within the density functional theory framework using the excited electron core-hole (XCH) approach. A simple structural model of a perfect c-BP crystal accurately reproduces the P L2,3-edge, however it fails to describe the broad and gradual onset of the B K-edge. Simulations of the spectroscopic signatures in boron 1s excitations of intrinsic point defects and the hexagonal BP crystal phase show that these additions to the structural model cannot reproduce the broad pre-edge of the experimental spectrum. Calculated formation enthalpies show that, during the growth of c-BP, it is possible that amorphous boron phases can be grown in conjunction with the desired boron phosphide crystalline phase. In combination with experimental and theoretically obtained X-ray absorption spectra of an amorphous boron structure, which have a similar broad absorption onset in the B K-edge spectrum as the cubic boron phosphide samples, we provide evidence for the presence of amorphous boron clusters in the synthesized c-BP samples.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...