Electrolytes at interfaces: accessing the first nanometers using X-ray standing waves†
Abstract
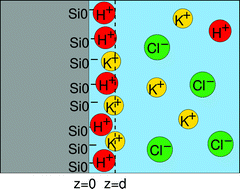
Ion–surface interactions are of high practical importance in a wide range of technological, environmental and biological problems. In particular, they ultimately control the electric double layer structure, hence the interaction between particles in aqueous solutions. Despite numerous achievements, progress in their understanding is still limited by the lack of experimental determination of the surface composition with appropriate resolution. Tackling this challenge, we have developed a method based on X-ray standing waves coupled to nano-confinement which allows the determination of ion concentrations at a solid–solution interface with a sub-nm resolution. We have investigated mixtures of KCl/CsCl and KCl/KI in 0.1 mM to 10 mM concentrations on silica surfaces and obtained quantitative information on the partition of ions between bulk and Stern layer as well as their distribution in the Stern layer. Regarding partition of potassium ions, our results are in agreement with a recent AFM study. We show that in a mixture of KCl and KI, chloride ions exhibit a higher surface propensity than iodide ions, having a higher concentration within the Stern layer and being on average closer to the surface by ≈1–2 Å, in contrast to the solution water interface. Confronting such data with molecular simulations will lead to a precise understanding of ionic distributions at aqueous interfaces.
- This article is part of the themed collection: Soft Matter at Aqueous Interfaces

 Please wait while we load your content...
Please wait while we load your content...