CO2 capture in ionic liquid 1-alkyl-3-methylimidazolium acetate: a concerted mechanism without carbene†
Abstract
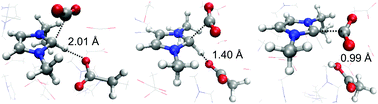
Ionic liquids (ILs) provide a promising medium for CO2 capture. Recently, the family of ILs comprising imidazolium-based cations and acetate anions, such as 1-ethyl-3-methylimidazolium acetate (EMI+OAc−), has been found to react with CO2 and form carboxylate compounds. N-Heterocyclic carbene (NHC) is widely assumed to be responsible by directly reacting with CO2 though NHC has not been detected in these ILs. Herein, a computational analysis of CO2 capture in EMI+OAc− is presented. Quantum chemistry calculations predict that NHC is unstable in a polar environment, suggesting that NHC is not formed in EMI+OAc−. Ab initio molecular dynamics simulations indicate that an EMI+ ion “activated” by the approach of a CO2 molecule can donate its acidic proton to a neighboring OAc− anion and form a carboxylate compound with the CO2 molecule. Analysis of this termolecular process indicates that the EMI+-to-OAc− proton transfer and the formation of 1-ethyl-3-methylimidazolium-2-carboxylate occur essentially concurrently. Based on these findings, a novel concerted mechanism that does not involve NHC is proposed for CO2 capture.

 Please wait while we load your content...
Please wait while we load your content...