Solution plasma synthesis of a boron–carbon–nitrogen catalyst with a controllable bond structure†
Abstract
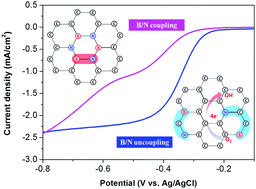
Synthesis of boron–carbon–nitrogen (BCN) nanocarbon with a controllable bond structure for enhanced oxygen reduction reaction (ORR) activity and durability was performed using a new method of discharge in organic solution mixtures named the ‘Solution Plasma Process’. Using selected precursors a new strategy for the simultaneous synthesis of nanocarbon co-doped with heteroatoms was found. The synergistic effect of N and B in an uncoupling bond state improved the formation of new active sites for the ORR performance by changing the electronic structure of the base carbon. Meanwhile, when B and N are bonded together, the BCN catalyst contributes to a reduced ORR activity by forming a balanced electronic structure in carbon. The BCN nanocarbon with an uncoupling bond state exhibits an enhanced ORR activity under alkaline conditions, with an onset potential of −0.25 V versus −0.31 V for B/N coupling and 3.43 transferred electrons during the ORR. Although the ORR activity of the B/N uncoupling nanocarbon was not as good as the typical Pt/C, the durability of this synthesized material (15.1% current decrease after 20 000 s of operation) was significantly better than that of the Pt/C catalyst (61.5% current drop under the same conditions). After the durability test, the increase of the chemical states containing oxygen was higher for Pt/C than B/N uncoupling.


 Please wait while we load your content...
Please wait while we load your content...