Novel isomorphism of two hexagonal non-centrosymmetric hybrid crystals of M(en)3Ag2I4 (M = transition metal Mn2+ or main-group metal Mg2+; en = ethylenediamine)†
Abstract
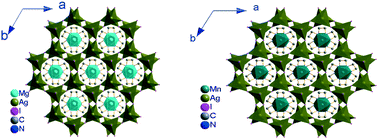
In this study, two isomorphic hybrid crystals of iodoargentate, M(en)3Ag2I4 (M = transition metal Mn2+ (1) or main-group metal Mg2+ (2) ion), have been prepared through in situ self-assembly in DMF solution at ambient temperature and characterized by microanalysis, IR spectroscopy, thermogravimetric analysis and powder X-ray diffraction and single crystal X-ray diffraction techniques. The magnetic properties of 1 have been investigated. The crystals of 1 and 2 crystallize in the hexagonal non-centrosymmetric space group P6322 with rather similar lattice parameters. The metal complex M(en)32+ (M = Mn2+ or Mg2+) with the symmetry of D3 point group plays a crucial role in directing the formation of the three-dimensional hexagonal non-centrosymmetric framework of {Ag2I42−}∞. To the best of our knowledge, the hybrid Mg(en)3Ag2I4 is the first example of a main-group metal complex used as a structure directing agent for the formation of a hybrid crystal structure, and this is also the first instance where isomorphic hybrid crystals are obtained using transition and main-group metal complexes. These findings can provide a basis for broadening the utilization of metal coordination cations/anions as structure directing agents in the design and construction of novel hybrid crystal structures in more effective ways.


 Please wait while we load your content...
Please wait while we load your content...