Polymorphism and solvates of 1-acetyl-3-(phenyl)-5-(1-pyrenyl)pyrazoline: the structures, thermal and optical-physical properties†
Abstract
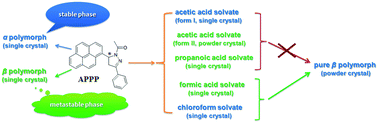
Single crystals of the β polymorph, formic acid and propanoic acid solvates of the title compound (abbreviation APPP) have been obtained. Single-crystal X-ray analysis revealed that the APPP molecules in the β polymorph construct the homochiral helical chains and the pyrene fluorophores adopt a monomer arrangement. Analogous structures were also found in the two solvates, while a small π-overlap exists between the pyrene fluorophores. Moreover, apart from the acetic acid solvate (form I) in a previous report, a new crystalline form (form II) was discovered through a rapid crystallization method and confirmed by PXRD patterns. The thermal and optical-physical properties of these crystals were investigated. All the three solvates could transform into the β polymorph after desolvation, but the pure β polymorph could be isolated only from the formic acid solvate. The optical properties are closely related to the pyrene fluorophore stacking modes and intermolecular interactions in the solid-state. The immobilization of pyrene fluorophores may be responsible for the high quantum yields.


 Please wait while we load your content...
Please wait while we load your content...