Particle anisotropy and crystalline phase transition in one-pot synthesis of nano-zirconia: a causal relationship†
Abstract
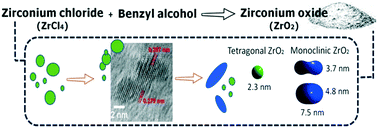
Crystalline phase evolution and morphological changes are strictly correlated phenomena during the growth of zirconia nanoparticles. In this work, the effects of synthetic variables, reaction time (up to 24 hours) and precursor concentration (0.16 and 0.5 M), of a one-step non-hydrolytic sol–gel route to zirconia are investigated. Zirconium tetrachloride (ZrCl4) is chosen as a zirconium oxide precursor to react in benzyl alcohol. At a low precursor concentration and a short reaction time, pseudo-spherical particles of size 2 nm with a narrow size distribution are observed by transmission electron microscopy (TEM). At this stage, mainly the tetragonal phase is detected. By increasing both the zirconium precursor concentration and reaction time, a broadening of size distribution is observed resulting from the growth of anisotropic particles. Concurrently, an increasing amount of the monoclinic is detected by X-ray diffraction and Raman spectroscopy. As a novelty, Rietveld investigations on electron diffraction ring patterns obtained by transmission electron microscopy are performed. This procedure allows the collection of comprehensive information about nanostructured particles in one-step analysis. The results derived from this analysis, together with the high resolution transmission electron microscopy (HR-TEM) data, consistently support the structural transition from pseudo-spherical tetragonal particles to rice-shaped monoclinic particles.


 Please wait while we load your content...
Please wait while we load your content...