Polymorphism in secondary squaramides: on the importance of π-interactions involving the four membered ring†
Abstract
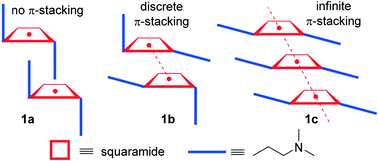
We report the X-ray solid state structures of four new squaric acid derivatives, i.e. three polymorphs of 3,4-bis((2-(dimethylamino)ethyl)amino)cyclobut-3-ene-1,2-dione (1a–c) and a co-crystal of compound 1 and resorcinol (2). All structures form interesting supramolecular assemblies in the solid state which have been analyzed using high level DFT calculations and molecular electrostatic potential (MEP) surface calculations. A combination of H-bonding and π–π stacking interactions of the cyclobutenedione rings are crucial for the formation of the supramolecular assemblies in the solid state. Moreover, unusual antiparallel CO⋯CO interactions observed in the X-ray structure of one of the polymorphs of 1 and the lp–π interactions between one oxygen atom of resorcinol and the squaramide ring in 2 have been characterized using Bader's theory of “atoms-in-molecules” (AIM).
- This article is part of the themed collection: Editor’s Collection: Polymorphism in Molecular Crystals


 Please wait while we load your content...
Please wait while we load your content...