Template-free synthesis of Co3O4 nanorings and their catalytic application†
Abstract
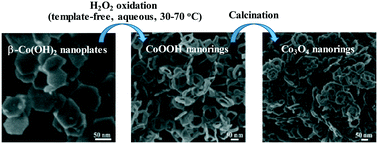
A facile, template-free and easily scalable approach has been developed for the preparation of Co3O4 nanorings, which can be divided into two stages: (1) formation of CoOOH nanorings and (2) high-temperature calcination of CoOOH nanorings in air. The key step for the synthesis of Co3O4 nanorings is the formation of CoOOH nanorings, prepared by the oxidation of β-Co(OH)2 nanoplates with H2O2 under strong basic conditions. The plausible growth mechanism governing the formation of CoOOH nanorings was correlated with Ostwald ripening. It was found that the dosage of H2O2 played a vital role in the formation of CoOOH nanorings. To a large extent, other experimental variables including the H2O2 concentration, Co2+ concentration, the molar ratio of Co2+ to OH− and the reaction temperature did not affect the preparation of the CoOOH nanorings. Furthermore, the as-prepared Co3O4 nanorings exhibited superior activity in comparison with Co3O4 nanoplates and commercial Co3O4 towards the catalytic decomposition of H2O2. The apparent rate constant of the as-prepared Co3O4 nanorings was 0.38 min−1, which is 2.2 times the value of Co3O4 nanoplates.


 Please wait while we load your content...
Please wait while we load your content...