Synthesis of tunable, red fluorescent aggregation-enhanced emissive organic fluorophores: stimuli-responsive high contrast off–on fluorescence switching†
Abstract
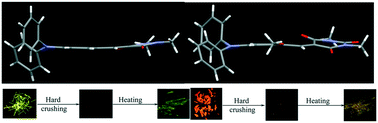
Triphenylamine (TPA) and N-methylbarbituric acid/indanedione-based donor–acceptor derivatives were synthesized and demonstrated molecular conformation and packing dependent tunable fluorescence from yellow to red in the solid state. TPA with N-methylbarbituric acid (BA-1) showed bright yellow fluorescence (λmax = 550 nm, Φf = 22.8%) whereas OCH3 substitution at the phenyl rings of TPA, (BA-2 and BA-3) produced strong red (λmax = 602 nm, Φf = 41.1%) and orange fluorescent solids (λmax = 582 nm, Φf = 19.1%). Indanedione acceptor dyes exhibited red to deep red fluorescence. ID-1 showed red fluorescence at 604 nm (Φf = 17.7%) whereas ID-2 and ID-3 showed fluorescence at 611 (Φf = 19.4%) and 636 nm (Φf = 14.1%) in the solid state. Solid state structural analysis revealed alteration of the molecular conformation and packing by OCH3 substitution which led to tunable fluorescence. BA and ID compounds showed turn-off fluorescence/substantially reduced fluorescence intensity upon hard crushing (Φf = 1.2 to 3.1%). Interestingly, heating of BA and ID crushed powders led to turn-on fluorescence/significant enhancement of fluorescence intensity (Φf = 5.6 to 22.5%). Powder X-ray diffraction (PXRD) studies indicated the conversion of the crystalline phase to amorphous and the amorphous phase to crystalline by hard crushing and heating. The reversible conversion of the crystalline phase to amorphous phase was responsible for the fluorescence switching of BA and ID. Computational studies have been performed to get an insight into the energy level modulation upon the change of the molecular conformation. Thus, we have presented the facile preparation of strong red fluorescent dyes exhibiting high contrast stimuli-responsive reversible dark and bright fluorescence switching in the solid state.


 Please wait while we load your content...
Please wait while we load your content...