Cyclometallated platinum(ii) and palladium(ii) complexes containing 1,5-diarylbiguanides: synthesis, characterisation and hydrogen bond-directed assembly†
Abstract
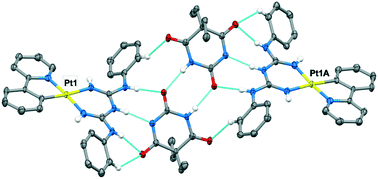
Complexes of the type [M(ppy)(big)], (where M = Pt(II) and Pd(II), ppy is 2-phenylpyridine and big is a 1,5-diarylbiguanide) have been prepared and characterised in solution and in the solid state. The big ligand possesses a DAD triple hydrogen bonding array that is designed to facilitate assembly of the complexes with complementary organic components. In DMF solution and in the solid state, the complexes are found to adopt a syn–syn conformer of the big ligand to allow hydrogen bonding of DMF molecules to pairs of NH donors. DFT calculations correctly predict this conformation, but only when DMF molecules are explicitly included in the calculation. The electronic spectral properties of the Pt(II) complexes are largely independent of the big ligand and are concentration independent in DMF solution, with TD-DFT calculations showing that the emission comes from the [Pt(ppy)] luminophore. In the presence of 1,8-naphthalimide and barbital derivatives, the big ligand adopts the alternate anti–anti conformer, which enables a triple hydrogen bonding motif. Notably, the two components adopt significantly non-planar orientations, a feature which will be important in their use as tectons for rational crystal engineering applications. Complexes of the type [Pd(ppy)(Hbig)]OAc, are also described, where the acetate anion is hydrogen bonded to the protonated Hbig ligand which adopts the anti–anti conformation.


 Please wait while we load your content...
Please wait while we load your content...