Structural aspects of partial solid solution formation: two crystalline modifications of a chiral derivative of 1,5-dihydro-2H-pyrrol-2-one under consideration†
Abstract
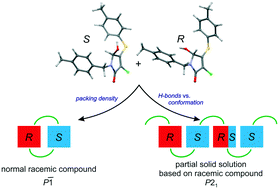
The purposeful change of crystallization conditions for rac-3-chloro-5-hydroxy-1-(4-methylbenzyl)-4-[(4-methylphenyl)sulfanyl]-1,5-dihydro-2H-pyrrol-2-one 1 leads to two different crystal modifications, namely, a racemic compound in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with Z′ = 1 (α-1) and a partial solid solution based on a racemic compound in the monoclinic space group P21 with Z′ = 4 (β-1). The first modification, α-1, is characterized by a higher density of the molecular packing in the crystal, while the second one, β-1, by a stronger system of hydrogen bonds and the presence of positional and substitutional disorder simultaneously. The analysis of the crystal structure of modifications α and β allowed us to define some structural aspects of the partial solid solution formation. Namely, the tendency to build a stronger hydrogen bond system enables the solution of enantiomers of 1 to be formed in the crystalline phase, whereas the propensity of the molecules to adopt a more favorable transoid conformation limits the solubility of the minor enantiomer.
with Z′ = 1 (α-1) and a partial solid solution based on a racemic compound in the monoclinic space group P21 with Z′ = 4 (β-1). The first modification, α-1, is characterized by a higher density of the molecular packing in the crystal, while the second one, β-1, by a stronger system of hydrogen bonds and the presence of positional and substitutional disorder simultaneously. The analysis of the crystal structure of modifications α and β allowed us to define some structural aspects of the partial solid solution formation. Namely, the tendency to build a stronger hydrogen bond system enables the solution of enantiomers of 1 to be formed in the crystalline phase, whereas the propensity of the molecules to adopt a more favorable transoid conformation limits the solubility of the minor enantiomer.


 Please wait while we load your content...
Please wait while we load your content...