Hierarchical CaCO3 particles self-assembled from metastable vaterite and stable calcite during the decomposition of Ca(HCO3)2†
Abstract
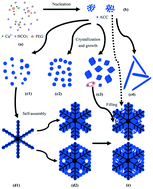
Calcium carbonate (CaCO3) was successfully synthesized via the decomposition of calcium bicarbonate (Ca(HCO3)2) solution in the presence of polyethylene glycol with different molecular weights (PEG 6000 and PEG 10000). The semi-quantitative characterization by XRD indicates that calcite is the major phase and vaterite and aragonite are the minor phases at different reaction times. SEM images show that the rhombohedral particle is the dominant morphology, together with some rod-like particles, a few spherical particles and some hierarchical structures, such as snow-shaped particles and spherical particles assembled from slice-like particles. SEM and TEM morphological images reveal that the snow-shaped particle consists of three parts, i.e. the hexagon or spherical center, the trunk and the filling. TEM crystal lattice images and electron diffraction patterns indicate that the trunk is composed of large vaterite crystal(s), while the filling consists of small calcite crystals. Based on the above results, the formation mechanism of snow-shaped particles is also proposed in the present work.


 Please wait while we load your content...
Please wait while we load your content...