An exploration into the amide–pseudo amide hydrogen bonding synthon between a new coformer with two primary amide groups and theophylline†
Abstract
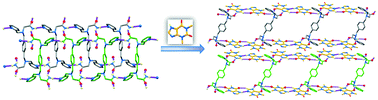
A cocrystal between a new coformer with two primary amide groups, 2,2′-((1,4-phenylenebis(methylene))-bis((pyridin-2-ylmethyl)azanediyl))diacetamide (2-BPXG), and theophylline (THP) was selected as a model system to (a) demonstrate the presence of a rare amide–pseudo amide hydrogen bonding synthon in it and identify further the structural features by single crystal X-ray diffraction, and (b) establish its relevant physicochemical properties through a comparison with the coformer by Fourier-transform infrared (FT-IR) spectroscopy, differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), differential thermal analysis (DTA), powder X-ray diffraction (PXRD), and hot stage microscopy (HSM). To the best of our knowledge, this is the first example where a coformer with two primary amide groups has been used to form the amide–pseudo amide hydrogen bonding synthon. The co-crystal (2-BPXG·4THP) crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with Z = 1, where the unit cell contains one 2-BPXG molecule and four THP molecules. Meanwhile, the coformer 2-BPXG crystallizes in the monoclinic space group P21/c with Z = 2 (the asymmetric unit contains half of the molecule). Surprisingly, only 2-BPXG (compared to other coformers with aliphatic spacers between the two alkyl nitrogen atoms), which does not form the amide–amide hydrogen bonding synthon within itself, paves the way for the formation of the amide–pseudo amide hydrogen bonding synthon R22(9) with THP. An overall 2D supramolecular network is formed in 2-BPXG through the interlinking of ladder-shaped layers (which are generated through strong hydrogen bonding between one of the N–H bonds and pyridine nitrogen) via strong hydrogen bonding between the other N–H bond and the carbonyl group of an adjacent molecule. On the other hand, the coformer with one primary amide group on each end generates a ladder-shaped layer in the cocrystal through hydrogen bonding interactions with THP molecules. These ladder-shaped layers are further connected via strong π–π (centroid to centroid distance: 3.68 Å) and weak C–H⋯O interactions between the THP molecules to form an overall 3D supramolecular network in the cocrystal. Hydrogen bond propensities, Hirshfeld surface analysis and quantitative crystal structure analysis of both coformer and cocrystal allowed us to understand the amide–pseudo amide hydrogen bonding synthon in detail.
with Z = 1, where the unit cell contains one 2-BPXG molecule and four THP molecules. Meanwhile, the coformer 2-BPXG crystallizes in the monoclinic space group P21/c with Z = 2 (the asymmetric unit contains half of the molecule). Surprisingly, only 2-BPXG (compared to other coformers with aliphatic spacers between the two alkyl nitrogen atoms), which does not form the amide–amide hydrogen bonding synthon within itself, paves the way for the formation of the amide–pseudo amide hydrogen bonding synthon R22(9) with THP. An overall 2D supramolecular network is formed in 2-BPXG through the interlinking of ladder-shaped layers (which are generated through strong hydrogen bonding between one of the N–H bonds and pyridine nitrogen) via strong hydrogen bonding between the other N–H bond and the carbonyl group of an adjacent molecule. On the other hand, the coformer with one primary amide group on each end generates a ladder-shaped layer in the cocrystal through hydrogen bonding interactions with THP molecules. These ladder-shaped layers are further connected via strong π–π (centroid to centroid distance: 3.68 Å) and weak C–H⋯O interactions between the THP molecules to form an overall 3D supramolecular network in the cocrystal. Hydrogen bond propensities, Hirshfeld surface analysis and quantitative crystal structure analysis of both coformer and cocrystal allowed us to understand the amide–pseudo amide hydrogen bonding synthon in detail.


 Please wait while we load your content...
Please wait while we load your content...