Morphology control of layered Bi11Fe2.8Co0.2Ti6O33 microcrystals: critical role of NaOH concentration and citric acid†
Abstract
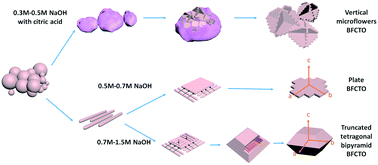
In this article, morphology control of Aurivillius Bi11Fe3Ti6O33 (4.5-BFCTO) microcrystals was investigated in detail in the hydrothermal process, where NaOH concentration and citric acid play a critical role. When NaOH concentrations are 0.3–0.5 M, 0.6 M, and 0.7–1.5 M4.5-BFCTO microflowers, microsheets and truncated tetragonal bipyramid particles formed, respectively. For the 0.3–0.5 M-sample, (Bi2O2)2CO3(OH)2 intermediate product, rather than (Na/Bi)7FexCoyTi5−x−yO21−δ of 0.6 M and 0.7–1.5 M samples, acts as a template and is responsible for the morphology change due to the competition between OH− and CO32− ions originating from the decomposition of citric acid. For the 0.6 M and 0.7–1.5 M samples, the morphology was controlled by the Gibbs free energy along the [001] direction. Besides, the dependence of magnetic properties on the morphologies was also investigated. This research on the morphology control could serve as guidance to potentially synthesize larger BFCTO single crystals and shed light on further designing nanodevices.


 Please wait while we load your content...
Please wait while we load your content...