Self-reversible thermofluorochromism of D–A–D triphenylamine derivatives and the effect of molecular conformation and packing†
Abstract
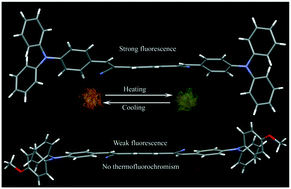
Triphenylamine (TPA) based donor–acceptor–donor (D–A–D) compounds were synthesized showing aggregation enhanced emission (AEE) and molecular conformation and packing dependent rare self-reversible thermofluorochromism in the solid state. 1, with TPA substituted with phenylenediacetonitrile at both ends, displayed a twisted molecular conformation, whereas OCH3 substitution at the ortho position of TPA (3-MTPA, 2) resulted in a twisted conformation at one end and a coplanar conformation at the other end. The 4-methoxytriphenylamine (4-MTPA) substituted acceptor (3) displayed a coplanar conformation at both ends of the acceptor. The highly twisted conformation of 1 led to strong fluorescence (16.8%) and the coplanar conformation of 3 resulted in weak fluorescence (1.6%) in the solid state. 2 showed moderate fluorescence (8.4%) in the solid state. Importantly, 1 and 2 exhibited self-reversible thermofluorochromism with heating and cooling, whereas 3 did not show any fluorescence switching with temperature change. Computational studies indicate that the molecular conformation and OCH3 substitution influence the HOMO–LUMO energy level of TPA derivatives.


 Please wait while we load your content...
Please wait while we load your content...