Molecular structure and hydrogen bond interactions of a paracetamol–4,4′-bipyridine cocrystal studied using a vibrational spectroscopic and quantum chemical approach†
Abstract
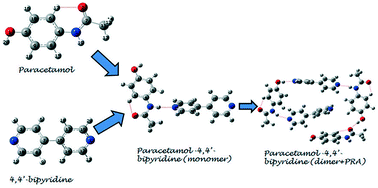
The purpose of the current study is to perform the structural and spectroscopic characterization of paracetamol–4,4′-bipyridine (PRA–BPY) cocrystal using infrared, Raman spectroscopy and density functional theory (DFT) calculations. To reveal the interactions between PRA and BPY, two models (monomer and dimer + PRA) of a cocrystal are designed and optimized using DFT with a 6-311G (d, p) basis set. An atoms in molecule study shows that the non-covalent interactions in particular hydrogen bonds involved in forming the cocrystal are moderate in nature. Natural bond orbital analysis of the second order perturbation theory of the Fock matrix suggests that interactions LP (1) N13 → π*(C15–O16) and LP (1) N56 → σ*(N13–H14) are responsible for the stabilization of the molecule.


 Please wait while we load your content...
Please wait while we load your content...