Diverse coordination polymers from a new bent dipyridyl-type ligand 3,6-di(pyridin-4-yl)-9H-carbazole†
Abstract
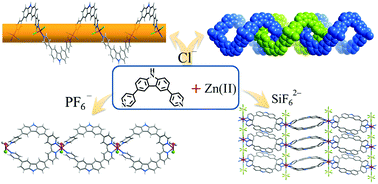
Reactions of a new bent dipyridyl-type ligand, 3,6-di(pyridin-4-yl)-9H-carbazole (dpc), with different Zn(II) salts yielded four coordination polymers, namely [ZnCl2(dpc)] (1), [Zn4Cl8(dpc)4]·1.44H2O (2), [ZnF(dpc)2(H2O)]PF6·2H2O·2DMF (3) (DMF = N,N-dimethylformamide), and [Zn(dpc)2(SiF6)]·guest (4). By virtue of the bent coordination geometries of both the tetrahedral Zn(II) ion and the dipyridyl ligand, as well as the termination effect of the Cl− counter-anion/ligand, 1 is a single-stranded helical chain. 2 is a supramolecular isomer of 1 containing the same bent ZnCl2(dpc) secondary building unit, but four of such units interconnect to form a molecular Möbius ring. More interestingly, the molecular Möbius rings in 2 mechanically interlock with their neighbours to form a curb chain. With weakly coordinating PF6− as a counter-anion, 3 is a cationic loop chain consisting of vertex-sharing rhombus molecular rings built from two metal ions and two organic ligands. 4 contains vertex-sharing loop chains similar to those in 3, but they are connected by SiF62− to form a neutral layered network possessing intralayer channels for gas adsorption.


 Please wait while we load your content...
Please wait while we load your content...