Phase transformation from α-Fe2O3 to Fe3O4 and LiFeO2 by the self-reduction of Fe(iii) in Prussian red in the presence of alkali hydroxides: investigation of the phase dependent morphological and magnetic properties†
Abstract
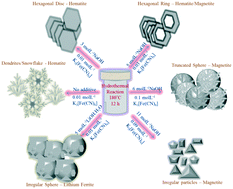
A facile surfactant and calcination free phase transformation from hematite (α-Fe2O3) to magnetite (Fe3O4) and lithium ferrite (LiFeO2) was achieved by the self-reduction of the Fe3+ ion in [Fe(CN)6]3− anions in the presence of alkali hydroxides (LiOH·H2O, NaOH and KOH) under hydrothermal conditions. It was noted that the CN− ion played a major role in the reduction of Fe3+ to Fe2+. Here, the alkali hydroxides acted as the reduction catalysts, as well as the structure directing agents. Hematite, magnetite and lithium ferrite exhibited different morphologies, such as dendrites, hexagonal discs, hexagonal rings, truncated spheres, irregular particles, irregular spheres, and assorted structures. The detailed reaction mechanism of the different phases and the growth mechanism of the different shapes have been investigated. The phase dependent magnetic properties have been explained based on the presence of a Fe2+ ion in the iron oxide crystal system.


 Please wait while we load your content...
Please wait while we load your content...