Ionic co-crystals of enantiopure and racemic histidine with calcium halides†
Abstract
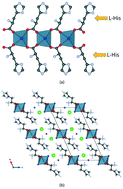
Ionic co-crystals (ICCs) of L- and DL-histidine with CaCl2, CaBr2 and CaI2 were prepared by mechanochemical and solution methods and were structurally characterized by either single crystal or powder X-ray diffraction methods. The L-histidine molecules bridge Ca2+ cations forming enantiopure ribbons in the homochiral crystals (L-His)2·CaX2·nH2O (X = Cl and Br n = 3, X = I n = 4), as well as in the partial dehydration product of (L-His)2·CaI2·4H2O, namely (L-His)2·CaI2·3H2O. In the racemic (DL-His)2·CaX2·4H2O cases (X = Cl, Br, X = I), molecules of both chiralities are accommodated in the coordination sphere of the Ca2+ cation forming ribbons with homochiral rims as in the enantiopure crystals. Intrinsic dissolution rate measurements show that the histidine-CaCl2 co-crystals have a much higher IDR with respect to both enantiopure and racemic histidine solids.


 Please wait while we load your content...
Please wait while we load your content...