Quantitative investigation of C–H⋯π and other intermolecular interactions in a series of crystalline N-(substituted phenyl)-2-naphthamide derivatives†
Abstract
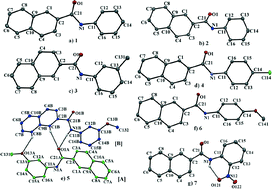
In this study, we have investigated the nature and characteristics of different intermolecular interactions present in a series of seven N-(substituted phenyl)-2-naphthamides. The seven structures comprise 2-naphthyl ring systems linked by amide bridges to N-bound phenyl 1, or substituted benzene rings 3–7, or in the case of 2, a cyclohexane ring. A common feature of the crystal packing for all but the o-nitro derivative 7 is the presence of a strong intermolecular N–H⋯O interaction. In the case of 7, the possibility of such an interaction is obviated by the formation of an intramolecular N–H⋯O hydrogen bond. An additional feature of the crystal packing for 1–6 is the significant roles that C–H⋯π contacts play in generating three-dimensional networks. In contrast for 7, the intramolecular N–H⋯O hydrogen bond precludes the formation of molecular chains but the planar nature of this molecule allows significant π⋯π stacking interactions to stabilize the packing.


 Please wait while we load your content...
Please wait while we load your content...