Ca2B2O5·H2O:Tb3+ hierarchical micro–nanostructures: formation and optical properties†
Abstract
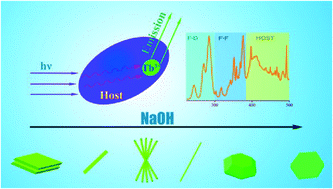
Ca2B2O5·H2O:Tb3+ hierarchical micro–nanostructures were synthesized using a facile hydrothermal method at 220 °C with addition of sodium hydroxide. The effects of sodium hydroxide, temperature and reaction time on the crystal structures and morphologies of the samples were explored. The results showed that these parameters play an important role in the dissolution–recrystallization controllable growth process, and the formation of the product was a result of the competition between NaOH and Ca2+ or boric acid. We also investigated the influence of the morphology and crystalline phase on the photoluminescence of Tb3+ ions in the borate micro–nanostructures. It has been found that the energy was first absorbed by the host of the borate crystal lattice and then transferred to Tb3+ leading to PL emission.


 Please wait while we load your content...
Please wait while we load your content...