Combination effect of ligands and ionic liquid components on the structure and properties of manganese metal–organic frameworks†
Abstract
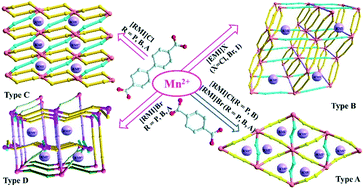
Ionothermal reactions of 1,4-benzenedicarboxylic acid (H2BDC) and 4,4′-biphenyldicarboxylic (H2BPDC) with Mn(OAc)2 resulted in 12 compounds divided into four kinds of structural models: [RMI]2[Mn3(BDC)3X2] (1–5, type A), [EMI]2[Mn3(BPDC)4] (6, type B), [RMI]2[Mn2(BPDC)3(H2O)3] (7–9, type C) and [RMI]6[Mn9(BPDC)9(HBPDC)2(OAc)4] (10–12, type D). A combination effect of ligands and IL components can be observed in the structural construction, which also is reflected in the properties of thermal stability and fluorescence. The decomposition temperatures of Mn–BDC compounds are higher than those of Mn–BPDC compounds. The decomposition temperatures decrease with the alkyl chain in [RMI]+, due to a +I inductive effect. The maximum emissions of compounds 1–5 and 6–12 located at ca. 410 or 407 nm are assigned to ILCT of H2BDC and H2BPDC ligands, respectively.


 Please wait while we load your content...
Please wait while we load your content...