Coordination polymers from a flexible alkyldiamine-derived ligand†
Abstract
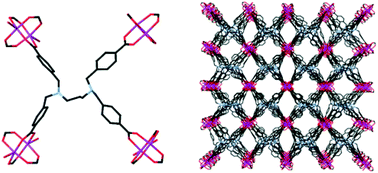
A traditionally good chelating motif has been incorporated into a ligand for the synthesis of coordination polymers with vacant amine sites. A novel tetracarboxylic acid diamine ligand, isolated as N,N,N′,N′-tetra(4-carboxybenzyl)-1,3-diaminopropane dihydrochloride trihydrate (H6L1)Cl2·3H2O, has been prepared, structurally characterised, and subsequently used in the formation of three new coordination polymers. Poly-[Cu2(L1)(OH2)2]·6DMF·3H2O 1 consists of (4,4) sheets formed by each L1 ligand coordinating to four copper(II) ‘paddlewheel’ dinuclear clusters. The amines of the ligand are non-coordinating. After solvent exchange and evacuation, 1 was found to adsorb approximately 23 cm3(STP) g−1 of CO2 at atmospheric pressure at 273 K. The isostructural compounds poly-[Zn(H2L1)(OH2)]·DMF·4H2O and poly-[Cd(H2L1)(OH2)]·DMF·3H2O 2Zn/2Cd are self-penetrating (10,3) coordination polymers containing square-shaped one-dimensional solvent channels. After solvent exchange it was found that 2Zn and 2Cd lost crystallinity, which accounts for the low N2 and CO2 uptakes observed.


 Please wait while we load your content...
Please wait while we load your content...