Nanostructured titanium phosphates prepared via hydrothermal reaction and their electrochemical Li- and Na-ion intercalation properties†
Abstract
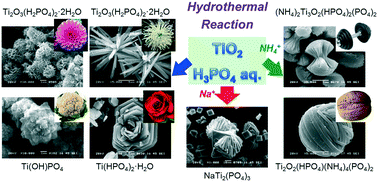
In this report, we demonstrate a facile and versatile methodology for synthesizing a series of titanium phosphates (TiPs) with various morphologies. The hydrothermal reactions of TiO2 in different concentrations of H3PO4 aq. yield 4 types of crystal structures with diverse shapes, such as nanoparticles, nanorods and nanosheets, which are assembled into a variety of flower-like morphologies. The dependence of the crystal phases and morphologies of TiPs on the synthesis conditions have been comprehensively investigated. This synthetic process can be further extended to other TiP derivatives incorporated with guest cations (e.g. NH4+ and Na+) simply by adding the corresponding phosphate salts to the starting composition, which leads to the morphological variation associated with the change of the crystal phase. The mechanism for the formation of the TiPs is discussed in terms of the stable Ti-based ion species relying on the pH value of the reaction solution. In addition, the correlation between the crystal structures and morphologies of TiPs and their electrochemical Li- and Na-ion storage behaviors are demonstrated as well, which discloses the different tendencies of morphological effects between the Li- and Na-ion systems.


 Please wait while we load your content...
Please wait while we load your content...